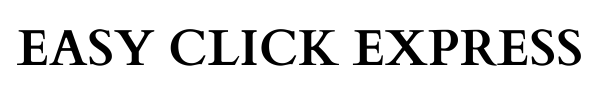A fifth-grader receives the FluMist influenza virus vaccine in Anaheim, Calif., in 2015.
Jeff Gritchen/Digital First Media/Orange County Register by way of Getty Pictures
conceal caption
toggle caption
Jeff Gritchen/Digital First Media/Orange County Register by way of Getty Pictures
The Meals and Drug Administration has permitted the primary flu vaccine that folks can administer to themselves at house.
The company on Friday gave the inexperienced gentle for individuals who have been screened to offer themselves the FluMist nasal spray, which could be ordered instantly from a web based pharmacy, skipping the necessity to go to a physician’s workplace.
It’ll nonetheless require a prescription from a physician’s workplace, nonetheless. It is anticipated to be accessible subsequent 12 months.
FluMist itself just isn’t new — the reside attenuated influenza vaccine has had FDA approval for greater than 20 years. However the means for adults to order the vaccine at house to manage to folks ages 2 to 49 is a breakthrough in comfort and entry to preventative care.
“Getting vaccinated every year is one of the simplest ways to forestall influenza, which causes sickness in a considerable proportion of the U.S. inhabitants yearly and will end in critical problems, together with hospitalization and demise,” stated Peter Marks, director of the FDA’s Middle for Biologics Analysis and Analysis.
“This approval provides another choice for vaccination in opposition to influenza illness and demonstrates the FDA’s dedication to advancing public well being,” Marks added.
Along with offering the comfort to get the vaccine delivered proper to your door, the nasal spray choice might encourage extra individuals who have fears of medical doctors or needles to inoculate themselves in opposition to the flu.
The FluMist nasal spray will probably be made accessible by means of a third-party on-line pharmacy, the place folks will full a screening course of to test eligibility. The FDA does advocate {that a} caregiver administer the spray to youngsters between the ages of two and 17.